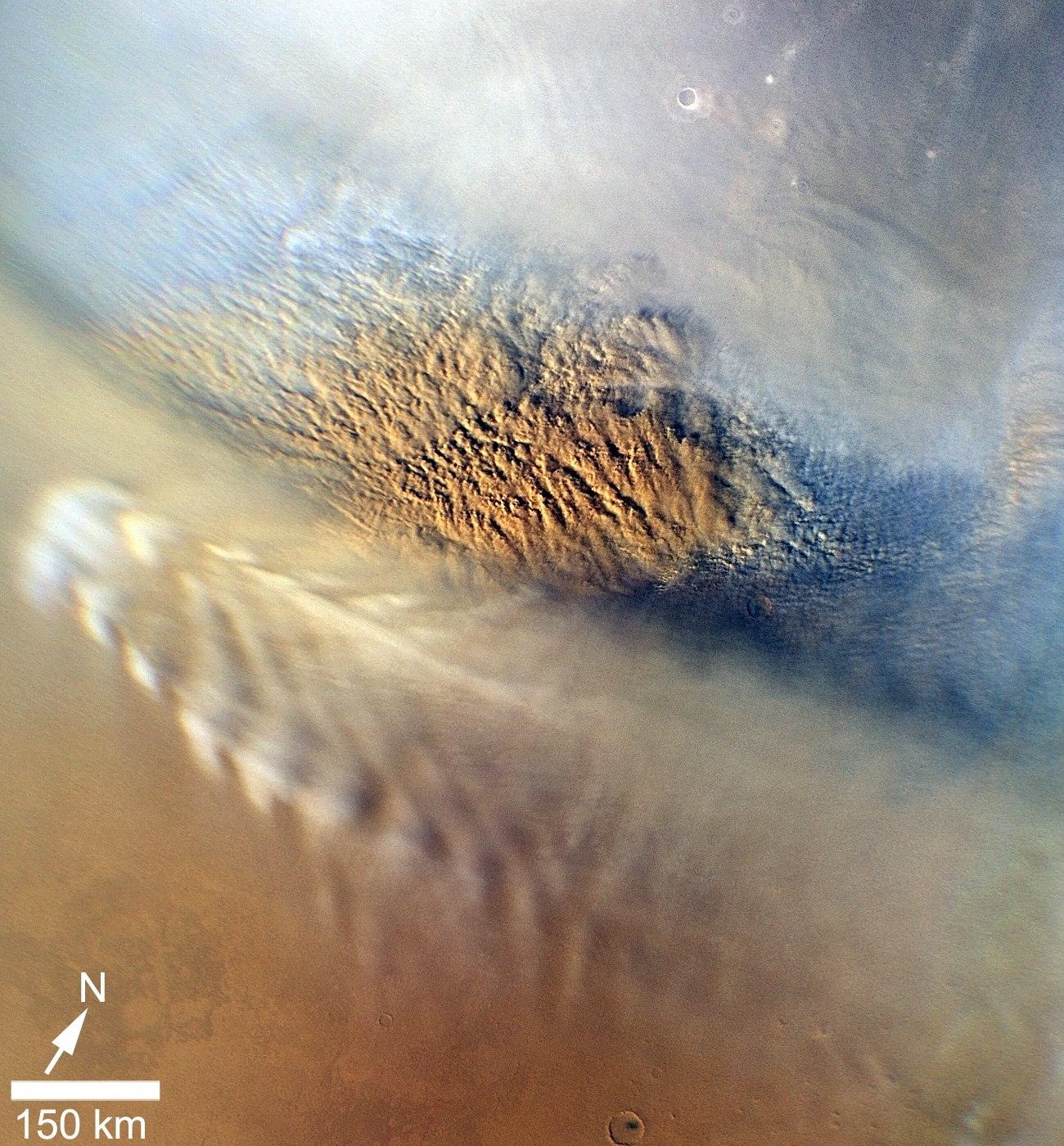
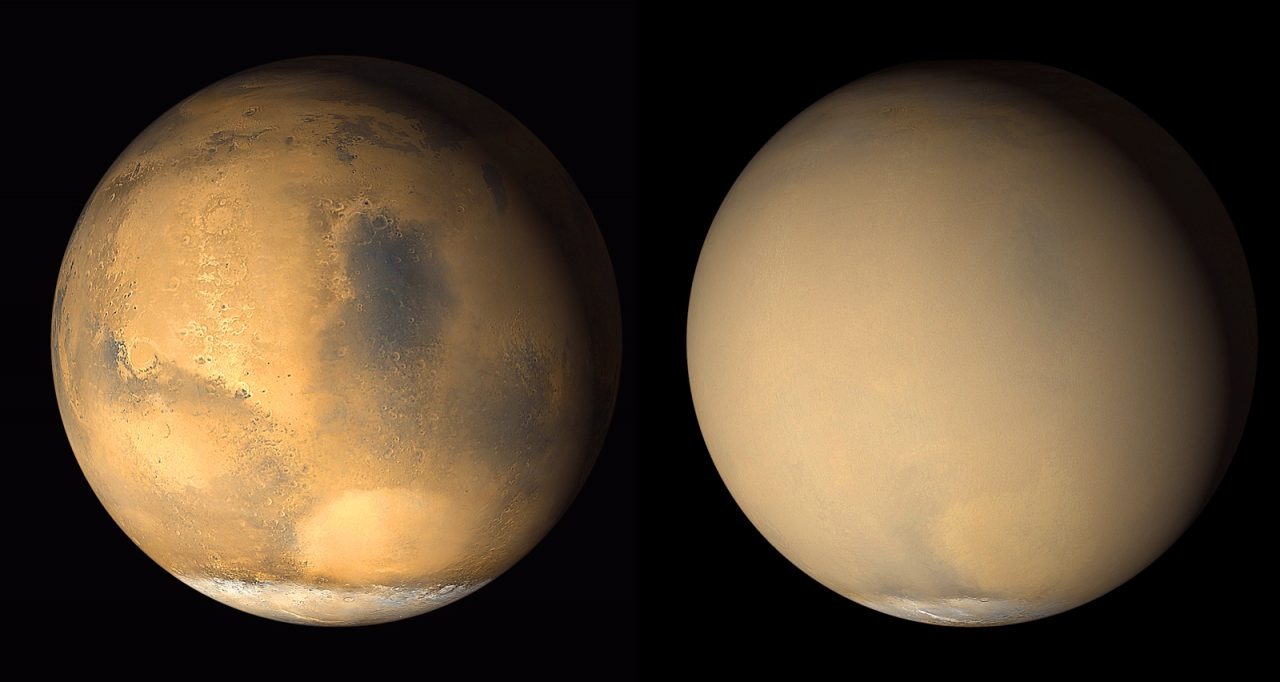
Dust storms on Mars pose a serious danger. While dust devils occur regularly, dust storms happen annually and can cover territories the size of a continent for weeks. Every three Martian years (5.5 Earth years) storms become so large-scale that they cover the entire planet and last up to two months. These storms play an important role in the dynamic processes forming the surface of Mars. But such cataclysms interfere with the exploration of the Red Planet. The storm of 2018 completed the mission of the Opportunity rover.

When Martian storms become particularly violent, friction between dust particles causes static electricity. According to research led by planetary scientist Alian Wang from Washington University in St. Louis, this electrification may be the main driving force behind the Martian chlorine cycle. Based on their analysis, Wang and her colleagues believe that this process may explain the discovery of large amounts of perchlorates and other chemicals by the rovers.
Dr. Wang led an international team of researchers from Texas Tech University, the University of Delaware, the McDonnell Center for the Space Sciences (MCSS) and the University of Oxford. An article describing their discoveries recently appeared in Geophysical Research Letters. In it, Wang and her colleagues demonstrated how an electric discharge caused by dust storms could be responsible for the decomposition of chloride salts, the formation of atmospheric chlorine and other chemical compounds on the surface (chlorates, perchlorates and carbonates).
Importance of Chlorine for Mars
On Mars, chlorine is considered one of the five “mobile elements”. The rest are hydrogen, oxygen, carbon and sulfur. Similar to the water cycle on Earth, chlorine is transported between the surface of Mars and the atmosphere in different forms. It exists in gaseous form in the atmosphere, while chloride deposits are on the surface — they resemble salt deposits on Earth.
The chloride deposits are probably dried-up remnants of salt water that once existed on the surface. There is a theory that they are formed as a result of the interaction between the surface and the atmosphere in the Amazonian period. This geological era continues today and, as scientists believe, it began with the end of the Hesperian period about 3 billion years ago, when Mars was still moving from a warmer, wetter environment to today’s dead and very dry.
Since there is no exchange between the surface and the atmosphere anymore, scientists wonder how the deposits of chlorine and chloride in the atmosphere can be related. In their new study, Wang (MCSS faculty member) and her colleagues demonstrated that electrical discharges caused by dust storms were an effective way to exchange chlorine between the surface and the air. The possibility that dust storms could be a source of reactive chemistry on Mars was first put forward during the Viking 1 and Viking 2 missions in the 1970s.
Experiments on Earth have shown that when electrostatic discharges interact with chlorine salts in an environment rich in carbon dioxide, for example, in the atmosphere of Mars, chlorine gas is released and perchlorates and carbonates can form. This was proved when the team subjected various ordinary chloride mineral salts to electrical discharges in conditions similar to those on Mars.

“Frictional electrification is a common process in our Solar System, with Martian dust activities known to be a powerful source of electrical charge buildup. The thin atmosphere on Mars makes it much easier for accumulated electrical fields to break down in the form of electrostatic discharge. In fact, it’s a hundred times easier on Mars than on Earth. The reaction rates are huge. Importantly, the released chlorine in a short-time mid-strength electrostatic discharge process is at a percent level. No other process that we know of can do this, especially with such quantitatively high yield of chlorine release,” said scientists at the University of Washington.
Lightning on Mars
Another interesting conclusion from this study showed what electrostatic discharges can be on Mars. According to Wang, the discharge will not look like a lightning flash on our planet. It looks more like the glow of the aurora borealis on Earth.
To date, no robotic mission on Mars has observed an electric discharge during a dust storm. From orbit, such lightning is not visible, and it is not possible to photograph the radiance on the surface, since the rovers stop their work during a storm.
Earlier we reported that Curiosity and Perseverance were not able to detect life on Mars.
According to Sciencealert.
Follow us on Twitter to get the most interesting space news in time
https://twitter.com/ust_magazine
