Scientists propose to solve problems with the processing of carbon dioxide and the provision of spacecraft and stations with energy and fuel using photoelectrochemical devices. According to the principle of action, they are similar to photosynthesis, which occurs in plant cells, but much more compact and economical.
Energy and oxygen in space
Scientists from the University of Warwick recently published an article in the journal Nature Communications, in which they described a way to provide spacecraft and stations on the Moon and Mars with oxygen, electricity and fuel. All this is proposed to be extracted using only one small device.
We are talking about devices that work on the basis of photoelectrochemical phenomena. The developers compare them with photosynthesis in terrestrial plants, although the developed installations will be much more efficient compared to them.
They have to solve three main problems that every autonomous environment with people will face: the presence of oxygen in the air, not carbon dioxide, electricity for instruments and fuel to move.
“Human space exploration faces the same challenges as the green energy transition on Earth: both require sustainable energy sources. With sunlight being so abundantly available in space, we have shown how this source could be used to harvest energy—much like plants back on Earth—for life support systems for long-term space travel. The technology could provide ample oxygen production and carbon dioxide recycling on both moon and Mars,” says Katharina Brinkert, one of the authors of the study.
Artificial photosynthesis
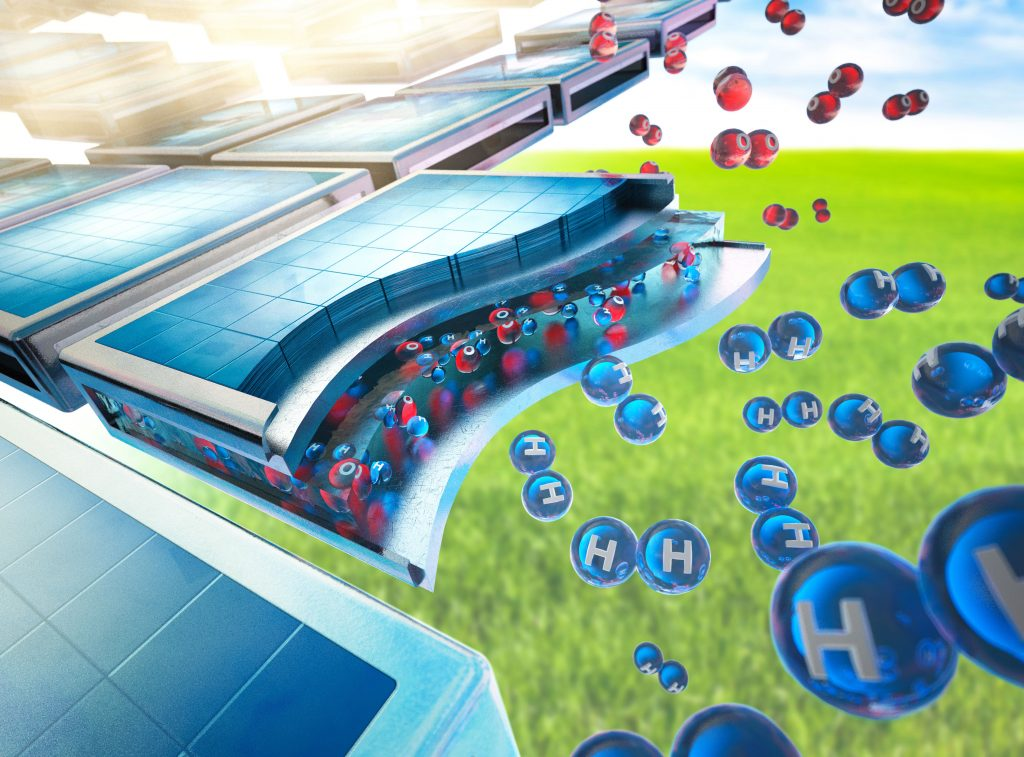
The article describes in sufficient detail what will happen in the devices under the influence of sunlight. First of all, the decomposition of water into oxygen and hydrogen will occur. In this case, the latter turns into ions, that is, electrons are torn off from its atoms.
Then the reaction goes in three ways. Some of the hydrogen ions attach electrons and turn into atomic hydrogen, which can be used as fuel or oxygen can be attached to it and water can be obtained again. Part of the hydrogen reacts with carbon dioxide and forms carbon monoxide and water.
Finally, a significant part of the hydrogen ions react with carbon dioxide and turn into methane and water. As a result of this process, a potential difference also arises, which can be used to generate an electric current by simply laying a wire between different parts of the devices.
According to phys.org
Follow us on Twitter to get the most interesting space news in time
https://twitter.com/ust_magazine
