It’s hard to say who first came up with the phrase “the entire periodic table of Mendeleev,” but now it is widely used by both the media and ordinary citizens to show that almost all known chemical elements are present in some products or mineral deposits. Technically, this is impossible because a significant portion of these elements only exist as radioactive isotopes with short half-lives, so they just “do not live long” under normal conditions. However, in the history of the Universe, there were times when this phrase had much more in common with reality. At that time, the periodic table consisted of only three elements, with the majority of the matter being composed of the first two: hydrogen and helium. All their company consisted of a small amount of the only metal known at that time — lithium. Due to modern technology, these “small amount” gradually become an integral part of our everyday life.
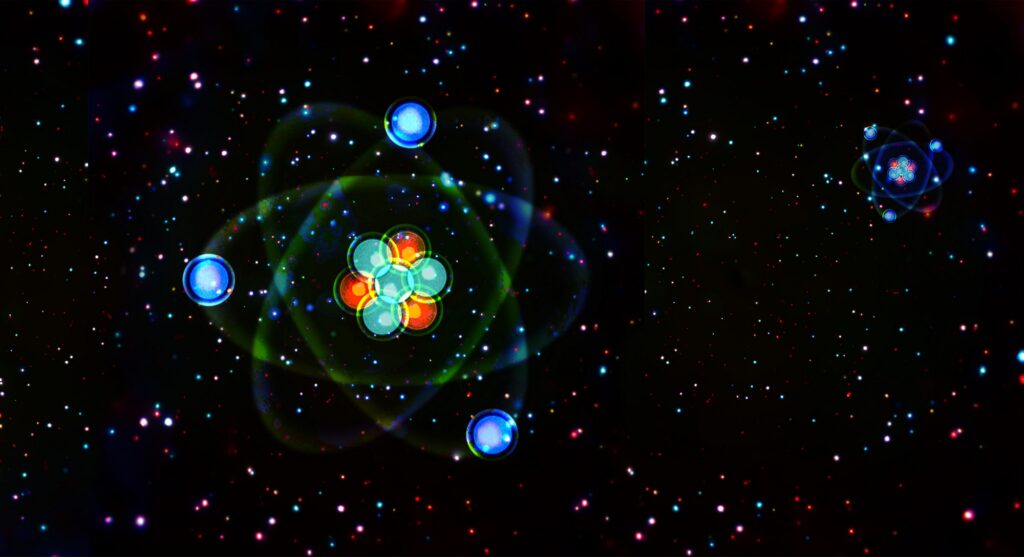
According to existing theories, the process of nucleosynthesis (the formation of the first atomic nuclei) began less than a second after the Big Bang. Initially, the main elementary particle was the neutron (n). However, “lonely” neutrons are unstable and decay into a proton and an electron, which respectively carry a positive and negative electric charge and together form the simplest atom of hydrogen (H). Some of the newly formed protons combined with neutrons to form deuterons – the nuclei of deuterium (D), a heavy stable isotope of hydrogen. This became possible around the 10th second after the “birth” of the Universe when its temperature and density had decreased enough for high-energy photons capable of destroying proton-neutron bonds to disappear. Almost all deuterons, in turn, quickly paired up, forming highly stable alpha particles – the nuclei of the main isotope of helium (He).
All the described reactions mainly ceased when our Universe “turned” 20 minutes old. At that point, its density became too low to provide a high enough probability of collision between atomic nuclei with the speed necessary for their further fusion. However, some processes of nuclear “aggregation” managed to go a little further, resulting in the appearance of heavier elements — isotopes of lithium (Li) with atomic masses of 6 and 7, as well as beryllium (Be) with a mass number of 7. The latter quickly decayed and transformed into the same lithium, which will become the main character of our story.
“The Lithium Paradox”
Modern theories describing the early stages of the universe’s evolution are supported by the relative abundance of chemical elements and their isotopes on large scales, but they also have some shortcomings. One of them is the so-called “lithium problem.”

All the “primordial” nuclei that formed after the Big Bang became the “raw materials” for thermonuclear synthesis in the cores of stars. Hydrogen (and its isotope deuterium) was merely consumed in these processes, while helium not only was consumed to form heavier elements but also synthesized from hydrogen. The amount of lithium, as previously believed, should also decrease because the reactions in which it is formed were unknown to scientists. Therefore, it should be rare in the universe, occurring in quantities proportional to the concentration of deuterium. Moreover, in older stars, its content should be higher than in younger ones. However, this contradicted the data obtained from spectral observations, which clearly showed that the younger the star, the higher its lithium concentration.
The mystery began to unravel when scientists started studying the interaction of cosmic rays—atomic nuclei (sometimes with considerable mass) moving at high speeds— with matter in interstellar space. It turned out that they were indeed capable of “breaking apart” the nuclei of other atoms they collided with, into fragments of various masses. Among them, a certain amount of lithium was found. However, their presence alone was not enough to explain the observed abundance of this element in the Earth’s crust and the universe. The real breakthrough in this direction came with the discovery in the 1980s of so-called lithium-rich giants.
Solar-type stars, toward the end of their life cycles when hydrogen in their cores has almost completely transformed into helium, start heating up (initiating thermonuclear synthesis of heavier elements from helium) and greatly increase in size, after which the temperature of their surface noticeably decreases. This transformation results in a red giant. In the case of our Sun, this will happen no earlier than in 4 billion years. This stage of a star’s “life” lasts significantly less than its total lifespan, but due to their high luminosity, we can see red giants even at great distances, which is why astronomers know a lot about such stars. And approximately one percent of them have an interesting feature: the concentration of lithium in them is hundreds or even thousands of times higher than what would be expected based on current understanding of stellar evolution.
At this point, we encounter one of the “white spots” in modern science: researchers still do not know during which processes such large amounts of lithium can be formed. The only thing known so far is that these processes can be initiated by the presence of certain heavy elements in the protostellar gas and dust cloud from which the star forms. Further analysis of the data revealed that our Sun, toward the end of its active existence, could also become such a “lithium giant.”
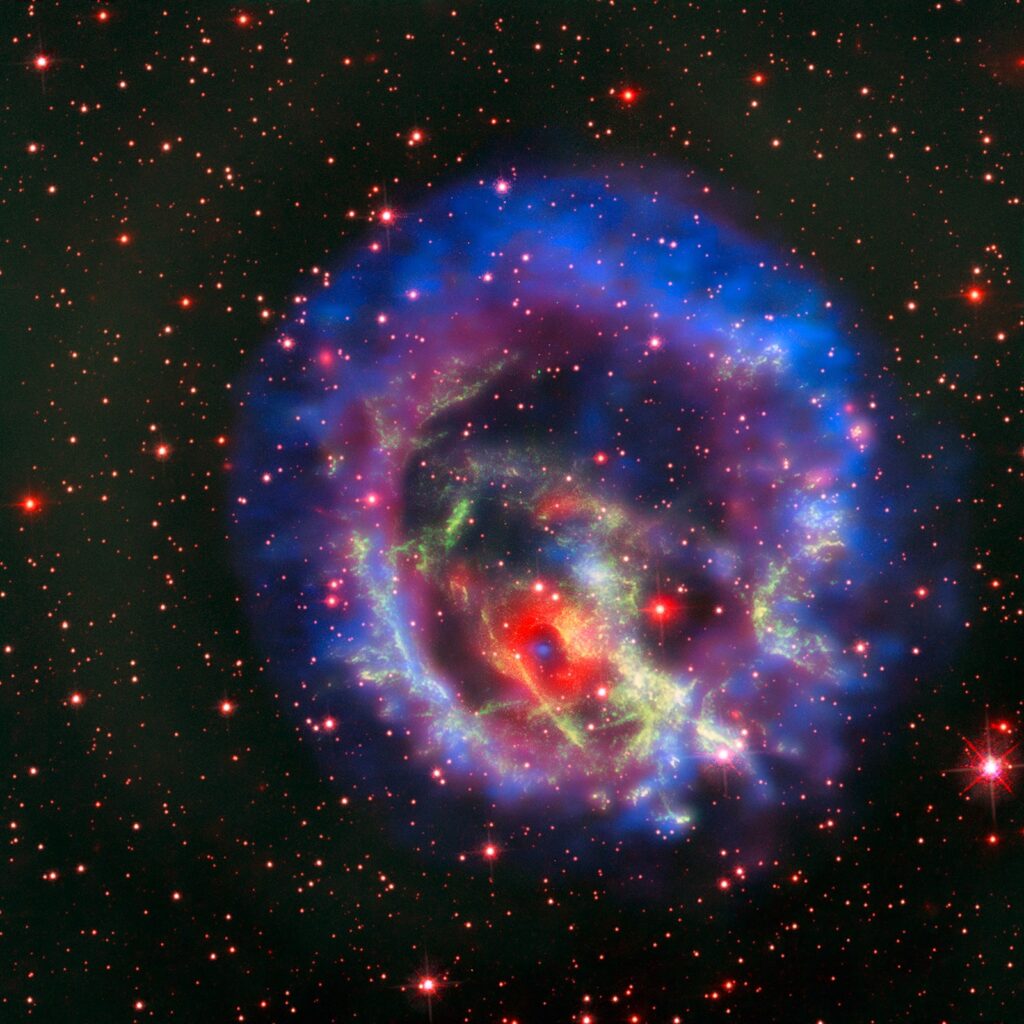
Before their final transformation into white dwarfs, these giants shed their outer shell into space, enriching the surrounding region with lithium. Thus, this metal becomes a component of interstellar matter and has the potential to participate in the formation of subsequent generations of stars and planets. It is likely that it eventually found its way to Earth through this process.
From a simple stone
Humanity became acquainted with lithium as a separate chemical element in 1817 when Swedish chemist Johan August Arfwedson isolated its hydroxide from the mineral petalite. The scientist quickly realized that he was dealing with a compound of an alkali metal. Two such metals were already known: sodium and potassium. However, both were found in the form of water-soluble salts, while the new element was part of an insoluble mineral, essentially an ordinary stone (in Greek, λίθος). Hence it received its name.

As soon as next year, lithium was obtained in metallic form through electrolysis (which is still used today). It was gradually discovered that lithium is also present in natural solutions of salts and in seawater. Its highest concentration was found in certain saline lakes in South America. It can be separated from other alkali metals by saturating a solution of their hydroxides with carbon dioxide: lithium carbonate, unlike sodium and potassium carbonates, is a sparingly soluble compound.
In the early years after its discovery, lithium was not deemed of any use until it was found that some of its salts, after careful drying, could effectively absorb water from the air and other gases. Later, lithium compounds found their place in medicine, glass production (adding lithium carbonate to the batch makes the glass more transparent to ultraviolet radiation), and organic synthesis. Lithium hydride was proposed as an efficient method of storing and generating hydrogen, and lithium oxide as a lightweight and compact carbon dioxide absorber.

In the 20th century, scientists began to learn about other valuable properties of lithium. In fact, it “unveils” a range of activity of metals in aqueous solutions. Its electrical potential was found to be the most negative, meaning that as an electrode in a chemical power source, it allows for the most efficient energy generation. Its performance per unit of mass is particularly impressive because lithium is also the lightest of all metals: its density is nearly half that of water at room temperature.

Unfortunately, this characteristic of lithium is also its main drawback: this metal is highly chemically active, instantly reacting with water and atmospheric gases such as oxygen and nitrogen (no other chemical element can directly interact with nitrogen). Therefore, all lithium energy sources must be carefully isolated from the surrounding environment. And here we come to the indispensability of lithium as a “space metal”: beyond the atmosphere, the possibility of such interaction can be completely eliminated, and thus, lithium-based batteries and accumulators are gradually becoming important components of the power systems of artificial satellites, manned spacecraft, and interplanetary probes.
The main structural material of spacecraft is aluminum. Recently, experts have been studying the possibility of creating aluminum alloys with the addition of lithium, which would have better mechanical properties, and most importantly, lower density, significantly optimizing the payload of launch vehicles.

But lithium also has its “military profession,” which has made it one of the most dangerous metals of modern times, on par with uranium and plutonium. The thing is, in order to ignite the thermonuclear reaction of helium nuclei synthesis, the deuterium and tritium must be under very high pressure, which is unattainable by current technologies. The problem was solved by using not gaseous hydrogen isotopes, but their compounds with lithium-6 — it is rumored that this solution was first proposed by Andrei Sakharov. Moreover, 6Li also actively participates in thermonuclear fusion, further increasing energy release during the explosion. However, just as purely peaceful inventions can get military professions, many military technologies are gradually being introduced into our everyday life. Perhaps in the future, reactors operating on lithium deuteride will become a powerful source of energy for interplanetary and interstellar research spacecraft.
Space Perspectives
The total amount of lithium on Earth is estimated to be over 300 billion tons, with moіе of it (230 billion tons) found in the water of seas and oceans. Unfortunately, its concentration there is very low, not exceeding 250 ions per billion. This greatly complicates the extraction of this element and forces geologists to pay more attention to lithium-bearing minerals such as petalite, lepidolite, spodumene, as well as to vast salt flats and certain sources of underground water. Chile possesses the largest economically recoverable lithium reserves, estimated at 7.5 million tons.

However, new data on the synthesis of the third element in stellar interiors and its distribution in protoplanetary gas-dust clouds provide grounds to hope that humanity may encounter even more promising lithium deposits in outer space. Mars and the subsurface oceans of Jupiter’s moons are potential sources of abundant lithium. Comets are also considered as possible “suppliers” (although they are much more difficult to capture). But all of this is more likely to be a matter of the distant future when humanity begins to inhabit the Solar System.

As for the present day, lithium compounds have already ventured beyond the atmosphere in the early days of the space age. Its peroxide and superoxide (Li2O2 and LiO2) are part of life support systems in many manned spacecraft, as they absorb carbon dioxide and water vapor from the air while releasing oxygen. There have also been considerations of using metallic lithium as an additive to liquid and solid rocket propellants. Its low density and relatively low melting point of only 180.5°C make it favorable. However, the jet stream generated by lithium fuel, is so hot that no existing structural materials can withstand it. Nevertheless, it is possible that this obstacle will be overcome in the near future. More significant challenges are the high cost of such fuel and its aggressiveness.
Due to the chemical activity of lithium, it can burn with the release of a large amount of heat in environments that are typically considered non-combustible on Earth, such as carbon dioxide gas. Lithium engines may become a propulsive force for research vehicles operating in the upper atmosphere of Venus.
Lithium (specifically, its most abundant isotope with an atomic mass of 7) and its alloys with other alkali metals have been proposed for use as coolants in nuclear reactors. Again, on Earth, these attempts are hindered by the highly flammable nature of such mixtures, which instantly ignite upon contact with air and burn with practically inextinguishable flames. However, on the Moon, for example, this danger is absent. Similarly, lithium can be used in so-called heat pipes for rapid heat transfer to the desired location. “Lithium pipes” are record holders in heat conductivity per unit mass.
From all that has been said, it is easy to understand that the illustrious history of the third element is only just beginning, and it will unfold not only on Earth but also in space. Some uses of lithium are even hard for us to imagine, but it can already be certain that it will be a worthy companion to the energy “pillars” of the next technological era — silicon and deuterium.
