We now know that the Sun is just one of many stars in the Galaxy. And all of them emit visible light and other electromagnetic waves due to thermonuclear reactions. Some elements are transformed into others, while part of the mass is transformed into energy. But the path to this knowledge was long and very difficult.
Why does the sun shine?
Since times immemorial, people have known that our luminary is the main source of light and heat on Earth, ensuring existence of plants and animals, so we, people, also do not die of hunger. It is not for nothing that legends about the end of the world were associated with the disappearance of the Sun in many mythologies.
However, the question of why the Sun shines not unanswered for many centuries, but in one way or another forbidden. Everything that happened in the heavens was considered the work of divine forces, and the most sacred celestial object was the Sun itself.

The very idea that the same physical laws apply in space as on Earth took root in the minds of scientists only in the 17th century. About the same time it became clear that the other stars are the same suns as ours, so everything that concerns it is also true for them.
The entire 18th century was devoted to finally finding out that energy does not arise from nowhere and does not disappear into nowhere. It only changes its from or is transmitted from one body to another. Finally, in the 19th century, science was ready to seriously muse upon the question of what source of energy feeds our Sun.
Why does the sun not go out?
The first attempt was made by the German physicist Robert Julius Mayer, who in 1848 calculated that if the source of the Sun’s energy were coal, it would burn out in just a few thousand years, taking into concideration the power of radiation observed now.
Although there are more calorific fossil fuels in nature, it was already clear to Mayer that no chemical reaction provides the required amount of energy. Therefore, he proposed an alternative theory: the energy of the Sun is released by meteorites burning up in its atmosphere. But in that case, they would have caused real hell on Earth as well. In addition, the mass of the luminary would have to increase rapidly and it would be impossible not to notice it.
This theory did not suit anyone, so in 1853 the English physicist William Thompson (better known as Lord Kelvin) and the German scientist Hermann Helmholtz proposed that the main source of the Sun’s energy is gravity. Compression of a constant mass of gas under the influence of its own gravity causes its heating.
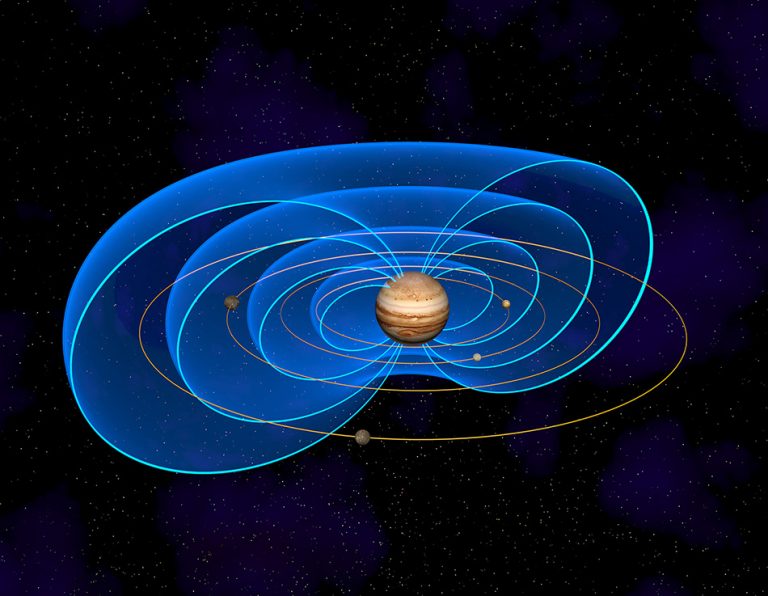
The Kelvin-Helmholtz mechanism can indeed lead to the release of energy. This is how it is produced by brown dwarfs and Jupiter-like giant planets. But with regard to the Sun, even the authors of the idea themselves calculated that it exists for no more than 20 million years and will disappear no later than in 15 million years.
This did not agree with the data of geology, which had established by that time that the age of rocks on Earth is measured in hundreds of millions of years. But scientists could not come up with anything better then, so the Kelvin-Helmholtz theory remained the most authoritative explanation of the mechanism of the luminosity of the Sun and stars until the beginning of the 20th century.
Elusive helium
Meanwhile, spectroscopy gave scientists a new way to determine the composition of any glowing object without actually “touching” it. Applying this method to the Sun, scientists immediately found many lines that belonged to elements not yet found on Earth.
One of them, helium, was discovered during a total solar eclipse on August 18, 1868. It got its name two years later after the Greek god of the Sun. It was conjectured that this element does not actually exist on Earth and that it may be somehow related to the glow, but nobody could even guess how exactly it happens, because the atom was still considered indivisible, which means that the very idea of thermonuclear reactions could not arise either.
Helium was discovered on Earth in 1881, but this discovery was not recognized at the time. Its presence on our planet was finally confirmed in 1898. This coincided with the beginning of research into the atomic nucleus, and this element played not the least role in it.
In 1906, Ernest Rutherford and other scientists studying the newly discovered radioactivity found out that α-particles are actually helium nuclei. This became one of the foundations of the modern planetary model of the atom and, as a result, the doctrine of fission and fusion reactions of atomic nuclei.
The sun as a thermonuclear reactor
Rutherford himself hypothesized that our star shines due to a radioactive decay reaction. This would significantly increase the possible time of its existence of such a luminary, but it remained unclear where on the Sun uranium is (its spectral features could not be detected for a long time). However, as early as 1920, a much more interesting and realistic idea appeared.
In 1920, Arthur Eddington noticed that four hydrogen nuclei, i.e. protons, have a total mass slightly greater than one helium nucleus. According to Einstein’s theory of relativity, the mass difference could turn into energy, and it should be enough to explain the glow of the Sun and the stars.

Both hydrogen and helium are present in the Sun. But it took physicists more than thirty years to understand how these elements transform into each other. Along with that, it became clear that not only energy is released in the depths of the stars, but also the chemical elements are formed that make up the planets and living organisms.
Proton-proton chain
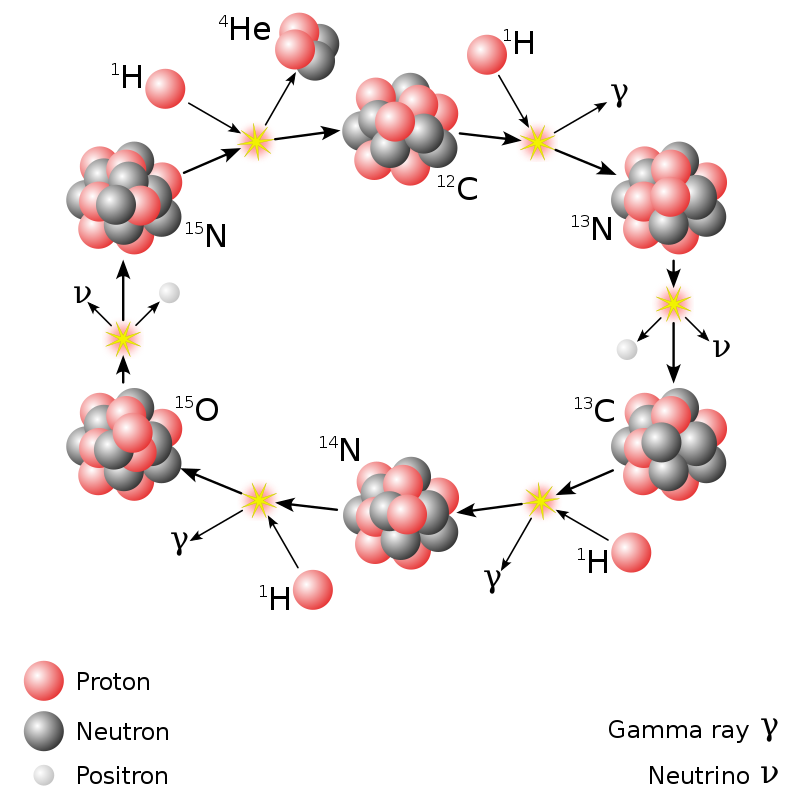
For stars like the Sun, the main way to get energy is the one described by Eddington. It is called the proton-proton chain and consists of several interconnected reactions. At the first stage, two protons combine to form a deuteron, the nucleus of a heavy stable isotope of hydrogen. At the same time, a positron, a neutrino and some energy are produced.
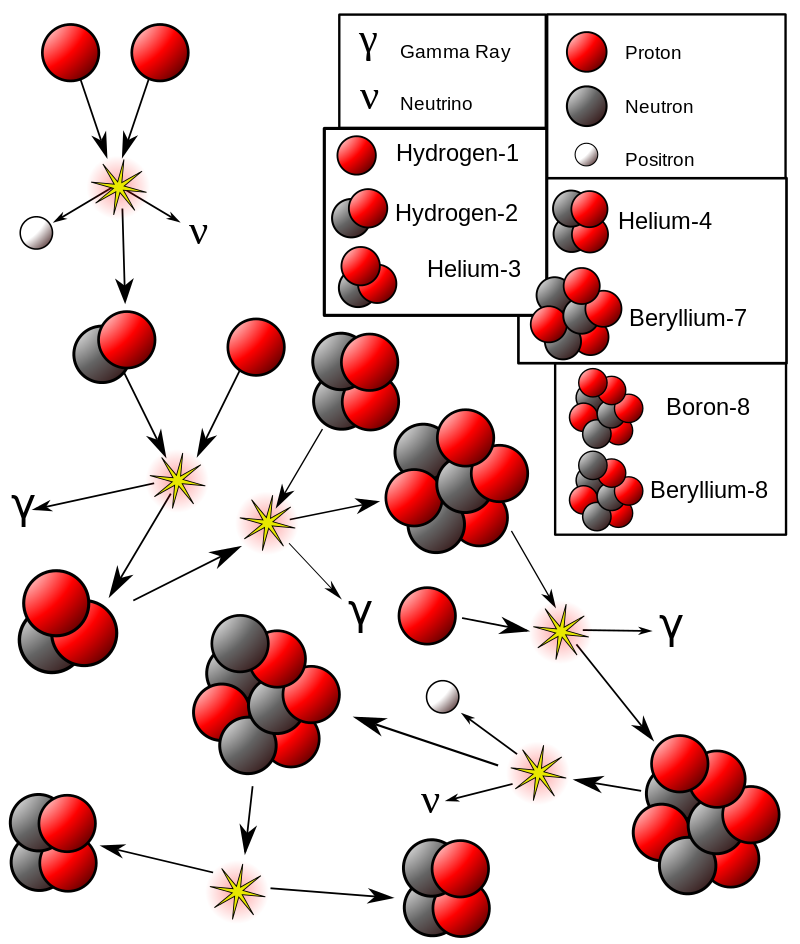
At the second stage, the deuteron attaches itself to a proton and turns into a helium-3 nucleus. At the same time, a gamma particle and much more energy is released. Finally, the final reaction involves two helium-3 nuclei fusing together and turning into a “normal” helium nucleus, two protons, and a considerable amount of energy.
In approximately one case out of 400, an electron is absorbed instead of positron emission at the first stage. At the same time, a little more energy is produced than in the usual version of the first stage of the chain.
But the cycle does not end there. Two helium nuclei can fuse to form beryllium. And lithium and boron are formed from it at extreme temperatures. In addition, as a result of the reactions of the proton-proton chain, oxygen, nitrogen and carbon can be formed, which are its final products.
CNO chain
The proton-proton chain is the main source of energy for stars as massive as the Sun or lighter. More massive luminaries, which are still on the main sequence (i.e. they have not yet used up their hydrogen reserves), use mainly the CNO chain.
This chain derived its name from carbon, nitrogen and oxygen, which are the main “actors” in it. They are the ones that attach protons to themselves, transforming into other elements and releasing energy.
The CNO chain releases significantly more energy than the proton-proton chain. However, the conditions for the occurrence of such reactions are much stricter. That is why they practically do not occur on the Sun.
“Burning” of heavy elements
When a star runs out of hydrogen “fuel” and begins to leave the main sequence, conditions for completely different thermonuclear reactions arise. As soon as the temperature in the star’s core exceeds 100 million Kelvin, a helium “burning” chain becomes possible. It does not release as much energy as reactions involving hydrogen, and the star can feed on it 100 times less time than previous patterns. Nevertheless, it prolongs its life a little.
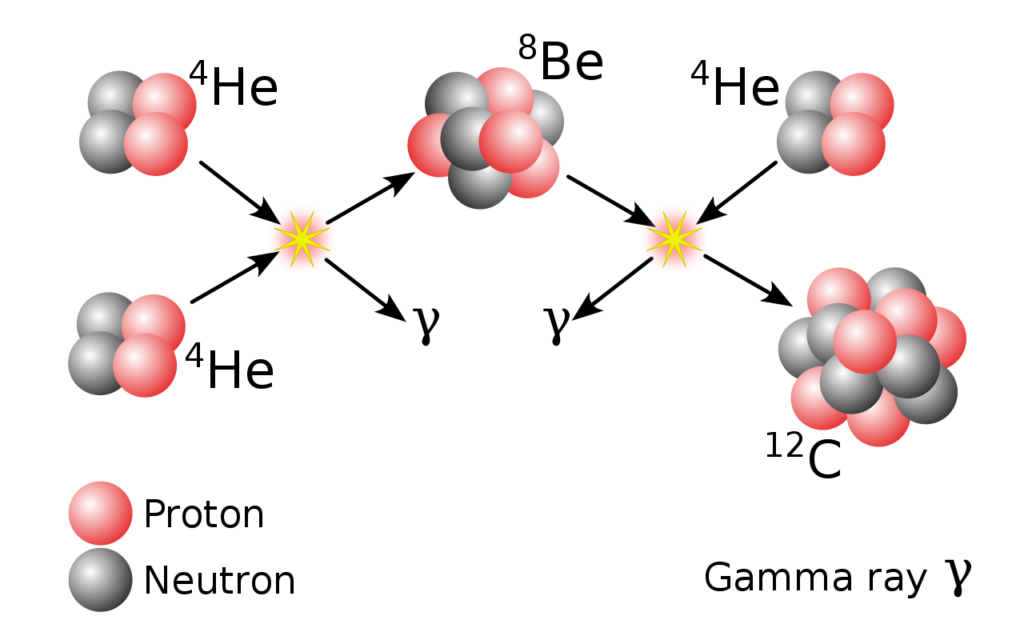
First, two helium-4 nuclei combine to form a beryllium-8 nucleus. Then the latter might grab another helium-4 nucleus turning into carbon-12, but it is unstable and usually does not have enough time to live for this.
However, as the temperature increases, more and more helium nuclei collide with each other forming carbon, which is stable. And it, in turn, begins to attach further helium nuclei to himself, turning first into oxygen, and then into neon.
That’s where it ends for most of the stars. But the largest of them, which are at least 10 times greater than the Sun by mass, are able to heat up to a temperature at which carbon, neon, oxygen and, finally, silicon begin to “burn” successively. Energy produced by these reactions becomes ever less, but they allow forming heavier chemical elements up to iron.

The rest of the elements of the periodic table are formed during much more extreme processes — for example, during supernova explosions. But there is no question of getting energy. Theoretically, quite exotic processes such as annihilation, that is, the reaction of ordinary matter with antimatter, can also be a source of the energy of stars. But so far no observational confirmation of the existence of such luminaries has been found.

