The mass media have long been full of dire warnings about the future shortage of energy resources, clean water, and arable land. But modern technological civilization needs other important components for its development, and some of them are hard to extract on the Earth. Will we be able to solve this problem developing other celestial bodies of our Solar System?

What are the “rare earths”
Rare earth elements (rare earth metals) — a group of 17 elements of the periodic table, which includes scandium (Sc), yttrium (Y), as well as a subgroup of 15 lanthanides: lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) and lutetium (Lu). The name “rare earth elements” was formed historically, because in the 19th century they were considered rare (in fact, this is not quite the case), and “earths” is an ancient name for poorly soluble oxides characteristic of these elements. In their free form, rare earth elements are typical metals of silver-white color and have similar chemical properties. Cerium, lanthanum and neodymium are in the greatest demand, and yttrium is indeed one of the rarest elements.

Geologists know almost 100 minerals containing rare earth elements, but phosphates (monacite and xenotime), fluorocarbonates (bastnesite and itrosynchisite), as well as loparite oxide are of practical importance as raw materials. Among industrial sources there are as well clay minerals of the weathering crust of granites.
Why are rare earth elements needed?
Today, rare earths are crucial for a number of key technologies in medicine and energy. They have found their place in more than a hundred industries. In particular, these elements are used as alloying admixtures for various steels and alloys, as well as in electronic devices, magnetic materials and ignition mixtures. In nuclear power plants, they are present as catalysts and hydrogen accumulators. Most of them are used in the form of mixed compounds, oxides and mixed metal (an alloy based on rare earth metals) as catalysts for cracking oil, as well as in the production of glass, ceramics and in metallurgy. These industries account for almost 70% of the total consumption of rare earth elements. They are also part of battery electrodes, magnets, contrast agents in MRI. It is difficult to overestimate the importance of these elements even at the household level, because they are important components in the manufacture of high-tech devices and various gadgets that are found in almost every home today: computers, mobile phones, tablets, laptops, etc. One such component is the neodymium magnets found in hard drives, loudspeakers and mobile phones. The use of rare earth metals made it possible to miniaturize all these devices — without them, smartphones would be the size of a shoe, and the weight of a laptop would be 10 kg.

Explored reserves of rare earth elements on Earth amount to approximately 120 million tons. Most of these reserves (about 45 million tons) are in China. In addition, major countries with large deposits of rare earths include India, Australia, Vietnam and Brazil. As for 2018, the production of rare earth metals in the world amounted to almost 170 thousand tons per year. That is, at the current rate of production, global reserves will last for 700 years. However, their consumption increases by 10-15% annually. Demand for these metals is increasing and beginning to outstrip supply as renewable energy becomes more and more important in the world. Metals such as neodymium and praseodymium are important in clean energy and high-tech fields, so they are in the spotlight, especially with the rise of electric and hybrid vehicles. Considering such a high demand and limited opportunities for their reuse, NASA experts suggested looking for rare earth elements on other planets and satellites — for example, on the Moon. According to scientists, there may be large deposits of these metals, the availability of which compensates for the cost of delivering the extracted raw materials to Earth.
Rocks of the Moon
The first samples of lunar rocks were brought to Earth in 1969, when American astronauts made the first landing on the Moon by Apollo 11 spacecraft. Currently, the largest collection of rock samples from our natural satellite, with a total mass of 382 kg, is stored in the Lunar Sample Laboratory Facility (LSLF) at NASA’s Lyndon B. Jonson Space Center (Houston, Texas, USA). It has been operating since 1979 and absorbed the material collected by astronauts during six expeditions to the Moon. The most valuable samples (2,200 units) are stored in glove boxes filled with inert gas (nitrogen) in a stainless steel vault. All instruments and containers are sterile. Chambers with samples are moved to other rooms of the Laboratory through special gateways. Each year, an independent panel of experts reviews new proposals from researchers, and the curators send out approximately 400 lunar samples to 40-50 scientists from around the world. Almost all samples are miniature — not heavier than one gram. After completing the research work, they must be returned to the Laboratory. Over the years of research, these samples have enabled scientists to understand the nature and origin of our satellite. It is believed that the Moon was formed about 4.5 billion years ago as a result of the collision of a Mars-sized planetoid with the young Earth, which resulted in a powerful emission into space of debris and a cloud of dust, which later “condensed” into a spherical object. It has been confirmed that most of the lunar craters were caused by asteroid and comet impacts rather than volcanism. A constant flow of meteorites, micrometeorites, as well as the influence of solar radiation changed the bedrock, creating a layer of fine-grained soil and dust (regolith) that covers the entire surface of our night luminary.

At an early stage of its evolution, the Moon was a liquid magmatic ball covered with a thin hard crust of lighter minerals. Over time, this crust turned into white anorthosite, which “floated” on the surface of the magma, forming the lunar highlands. Later, as a result of the solidification of magma, basaltic rock arose. Anorthosite and basalt are the main native rocks of our satellite. Another common lunar rock, breccia, is the fragments of other rocks cemented together by the heat of volcanic activity. Anorthosite (plagioclaseite) is an igneous rock consisting mainly of calcium-rich calc-alkaline feldspar (usually labradorite, less often — andesite or bitovnite) with a small content of colored minerals — olivine, pyroxene, titanomagnetite, apatite, etc. On Earth, deposits of titanium, chromium, platinoids and, in some cases, rare earth metals are confined to anorthosite magmatic complexes.
Lunar deposits of rare earth metals
Interest in our natural satellite, which began to fade little by little in the 80s of the last century, was “warmed up” by the discovery of signs of the presence of deposits of water ice and other frozen volatile compounds near the lunar poles. The presence of water on the Moon would significantly facilitate its further colonization and development of minerals on its surface.
The first automatic device in the new millennium was sent to the Moon by the European Space Agency (ESA) back in 2003. The SMART-1 probe was launched on September 28, 2003 and worked in a selenocentric orbit for almost three years, after which it was transferred to a collision course with the lunar surface. The example of the Europeans was followed by Japan, India and China. The most successful lunar mission still in operation today is the American LRO (Lunar Reconnaissance Orbiter) probe, which has been operating in a selenocentric orbit for 11 years and transmits to Earth the most detailed images of the surface of our satellite, as well as many other scientific data.
Plans for the landing of people on the lunar surface are currently being actively developed by the United States (in cooperation with the European Union) and China. NASA is preparing a new landing of a man on the moon in 2025 (mind that the last time American astronauts visited the Moon in December 1972). The mission is named Artemis and is aimed at delivering the first woman to the surface of our satellite. The goal of the expedition is also the south lunar pole, where there are deep craters, the bottom of which never gets sunlight, and areas that are illuminated almost constantly. Probes powered by solar batteries could function in such areas.
The management of NASA believes that the implementation of plans for exploration and extraction of rare earth elements on the Moon is quite possible in the coming years, given the huge amount of investments in the space industry. It is worth noting that funds are already coming not only from states and nations, but also from powerful private companies (SpaceX, Blue Origin and Virgin Orbit).
The US also has plans for a long-term presence of astronauts on the Moon. Companies Boeing and Lockheed Martin have already entered into an agreement with NASA to create a space launch vehicle and capsule that will work for the lunar exploration program. SpaceX and Blue Origin are already working on creating spacecraft that will carry people and cargo to the Moon. Thanks to the involvement of private companies, it is expected that NASA’s expenses for the implementation of this program will not exceed 20 billion US dollars. Blue Origin, founded by the world’s richest man (as of 2019), billionaire Amazon CEO Jeff Bezos, is the lead contractor in this partnership. In May 2019, the head of the company presented the project of a landing spacecraft, which was named Blue Moon. NASA’s road map for landing a man on the moon in 2025 envisages the construction of a mini space station in lunar orbit, named Gateway. The lunar lander, provided by a commercial organization, will be sent to the Gateway Station, where it will await the arrival of a team of NASA astronauts.
Meanwhile, ESA specialists are developing technologies that would allow building a lunar base using 3D printing. The British architectural bureau Foster+Partners joined this process. The possibility of using lunar soil as the main building material is being considered. The design of the suspended dome has already been developed, which will have a cellular structure to protect against micrometeoroids and cosmic radiation, as well as an inflatable shelter for astronauts. According to the architects, the hollow structure of the buildings, reminiscent of bird bones, will provide an optimal combination of strength and weight. 3D printing technology should simplify the process of exploring the Moon, as it will not require material and technical supply from the Earth. The British company Monolite has already presented a D-Shape printer capable of spraying a binding solution from a construction material similar in physical properties to sand. Three-dimensional “printouts” are created layer by layer. The company usually uses its printer to make sculptures and artificial coral reefs that help protect beaches from sea waves. In lunar conditions, they plan to mix regolith with magnesium oxide and use this mixture as a building material for 3D printing. Further processing with a special solution should turn the finished structure into a solid material similar to stone.
So far, experimental studies have been successfully conducted on the possibility of 3D-printer operation in conditions close to those on the Moon. Basalt rock from a volcano located in the central part of Italy was used as an analogue of regolith. The laboratory also recreated the conditions of the space vacuum. Currently, further research is underway aiming overcoming temperature limitations (3D printing works best at +20°C) and creating safe conditions for astronauts, primarily protecting them from inhaling lunar dust, whose sharp particles can damage the lungs and other respiratory organs.
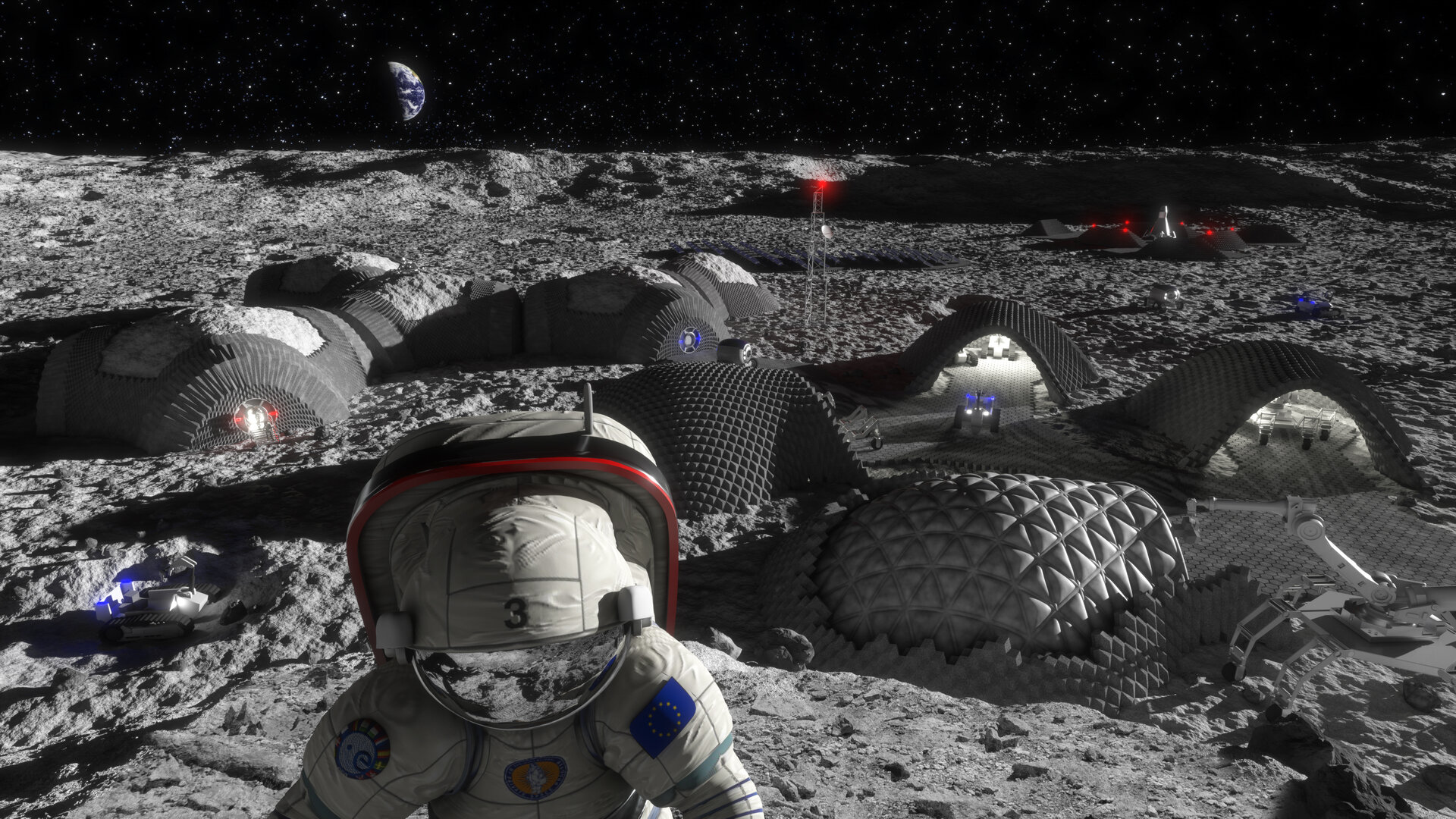
Thus, 50 years after the first human landing on the surface of the Earth’s natural satellite, we will witness another “boom” of lunar research. And perhaps soon each of us will have a “piece of the Moon” in her or his pocket, because the use of rare earth elements of extraterrestrial origin for the manufacture of smartphones will become quite a commonplace.

