In our daily practice, we consider diamonds to be precious stones. First of all, this is due to the fact that they are extremely rare on Earth. Even nowadays, their annual production does not exceed 30 tons (0.02 carats per inhabitant of the planet). But there are worlds in the Solar System where they literally “rain down”. However, there is also bad news: if we ever manage to catch even a drop of this “diamond rain”, it will not happen very soon.

Of the large planets in the Solar System, only two have not yet had artificial satellites. We are talking about the “ice giants” Uranus and Neptune. We know about their appearance thanks to photographs taken from the flight path by the Voyager 2 automatic spacecraft back in 1986 and 1989. But scientists have not yet managed to look “deeper”. We can only make theoretical assumptions about what is happening in the interiors of these planets.
How did the term “ice giants” originate
The fact is that Jupiter and Saturn, which are closer to the Sun, are almost entirely composed of light gases — hydrogen and helium. According to spectral studies, these elements also predominate in the atmospheres of Uranus and Neptune. But their main components are water, methane and ammonia. These matters, except methane, for the most part are in a solid form, which has little resemblance to the water or ammonia ice known to us. The reason for this is the incredibly high pressure in the interiors of the two most distant planets. It is believed that in their centers there should be an iron-stone core, heated by gravitational compression to at least 7 thousand degrees Celsius.
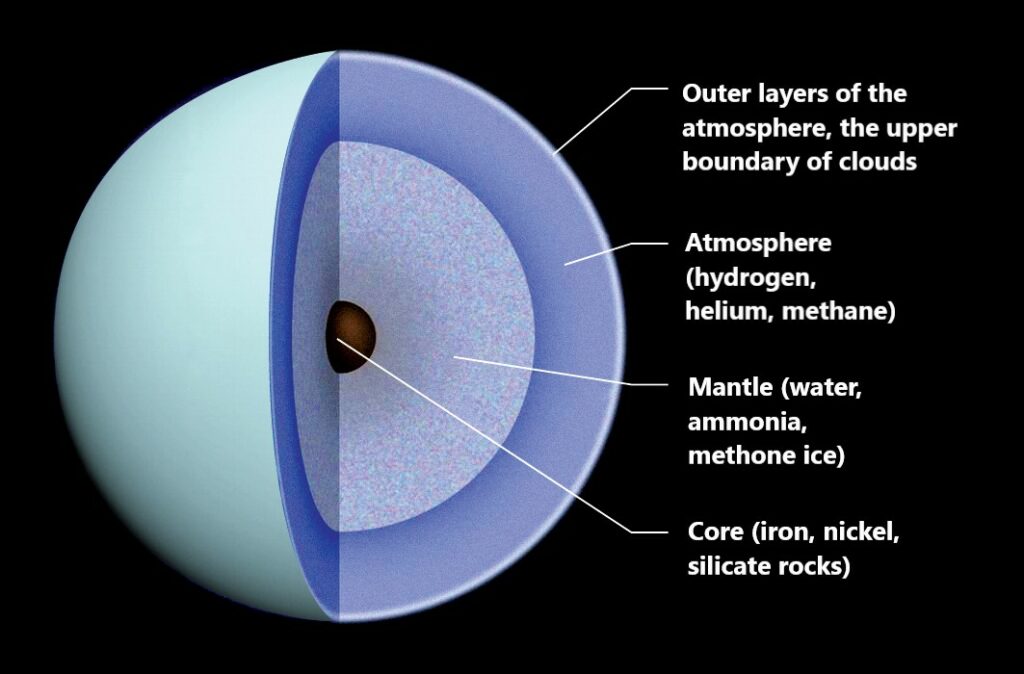
In the clouds of the ice giants, a fierce cold reigns — about 200 degrees with a minus sign. As they approach the core, their gas shell heats up. In parallel, the pressure is also increasing, forcing matter to move into exotic quantum states, due to which these planets practically have no solid surface. Although in fact, the described picture exists only in the calculations of specialists. To build it, astronomers and planetary scientists used a small amount of data obtained during ground-based observations. They were also helped by the information transmitted by the Voyager 2 probe. Then complex methods of mathematical modeling and laboratory experiments with high pressures and temperatures were involved. Their goal was to reproduce the internal environment of Uranus and Neptune. What did the scientists find out as a result?
Of all the chemical compounds that make up the outer planets, methane has the lowest boiling point. That is, it is difficult to convert it into a solid or some other “compacted” state even under high pressure. At a depth of about 7 thousand km from the upper boundary of the clouds, where this pressure reaches 200 thousand atmospheres, the temperature is already rising to about 1750 °C. If the water and ammonia molecules remain stable at this temperature, then methane begins to gradually break down into its components — carbon and hydrogen. The latter, as the lightest of the gases, rises into the upper atmospheric layers. But interesting things are beginning to happen with carbon.
Individual “free” carbon atoms begin to unite with each other in chains. Then complex spatial “constructions” are formed from them, forming, in the end, the crystal structure of a diamond. As we know, diamond is not only the hardest substance known. In addition, it has the simplest composition of all minerals, because it contains only carbon, as well as minuscule amounts of impurities, sometimes coloring natural diamonds in different colors.
How diamonds are formed
Solid carbon is most often formed during the thermal decomposition of organic matter. In terrestrial conditions, it condenses into large “molecular plates”. Then they are combined together into the most famous kind of this chemical element — graphite or carbon black. But under high pressure and at high temperatures, it crystallizes in the form of its precious modification — diamond. And it is precisely these temperatures and pressures that are present in a certain layer of the atmospheres of Uranus and Neptune.

The idea of a “diamond rain” arose long before the Voyager 2 probe flew near the outer planets. At first it seemed exotic, but later scientists came up with a way to test it. Modern technology does not allow us to dive into the depths of the “ice giants” and transmit information from there. But it is quite possible to partially reproduce their environment in the laboratory. For example, appropriate pressures and temperatures can be achieved for a short time by irradiating solid carbon and hydrogen compounds with a powerful laser. As a “target”, ordinary expanded polystyrene, better known as “foam”, was chosen. The result of the experiment was diamond nanoparticles — exactly what was expected to be obtained.
In the Uranian and Neptunian interiors, the “necessary” pressures and temperatures are constantly present. Therefore, diamond crystals there can grow to much larger sizes. Having a much higher density than their gaseous surroundings, they begin to fall on the core of the planet. At some depth, it gets so hot there that even crystalline carbon begins to evaporate. Obviously, there must be a way by which it gets to the overlying layers of the atmosphere again, where it reacts with hydrogen and forms methane again — otherwise the reserves of this gas on the ice giants would have dried up long ago. Planetary scientists have already proposed several similar mechanisms. Further research will show which of them takes place in reality.
According to www.space.com
Follow us on Twitter to get the most interesting space news in time
https://twitter.com/ust_magazine

