The ozone layer is a part of the Earth’s stratosphere containing up to 90% of ozone, which is a form of oxygen. It plays a crucial role in absorbing most of the hard ultraviolet radiation. As the effects of our industry began to deplete this layer, and we became aware of it in the 1980s, a lot of effort has been put into addressing the issue and reducing the damage.

The ozone layer
When talking about the negative effects of human activity on the environment, the ozone layer often comes up as a heated topic. The ozone layer shields us from invisible threats, yet remains imperceptible as we continue to deplete it through our own actions.
As a result, this topic has long been clouded in myths and misconceptions. Even though there’s plenty of scientific evidence, many still deny the reality of the problem, turning it into something that feels more like a theological debate.
The discovery that the upper atmosphere has a layer that absorbs most of the hard radiation was made in 1913, long before environmental concerns became the focus. Spectroscopic analysis at the time clearly identified ozone as the key to absorbing these rays.

How does ozone protect us?
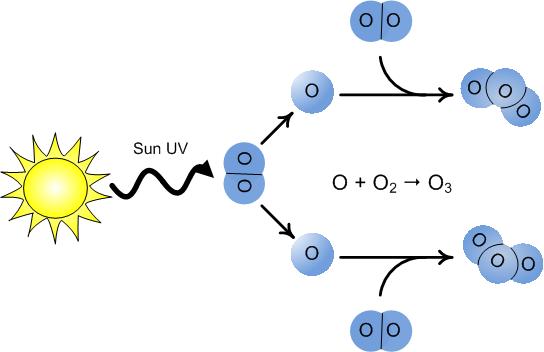
So what exactly is ozone? It’s a form of oxygen, or allotrope, that forms when a regular oxygen molecule with two atoms splits apart. These atoms then recombine to create a molecule made of three oxygen atoms, known as ozone.
Ozone can form through various processes. For example, it can be created when an electric current passes through the air. That distinctive smell you notice during thunderstorms or around electronic equipment is actually ozone.
Most ozone, however, is formed in the stratosphere, 12 to 25 miles above the Earth’s surface. At this altitude, charged particles collide with oxygen molecules, causing them to split apart and create ozone. This ozone then absorbs UV rays, breaks down, and the cycle repeats itself.
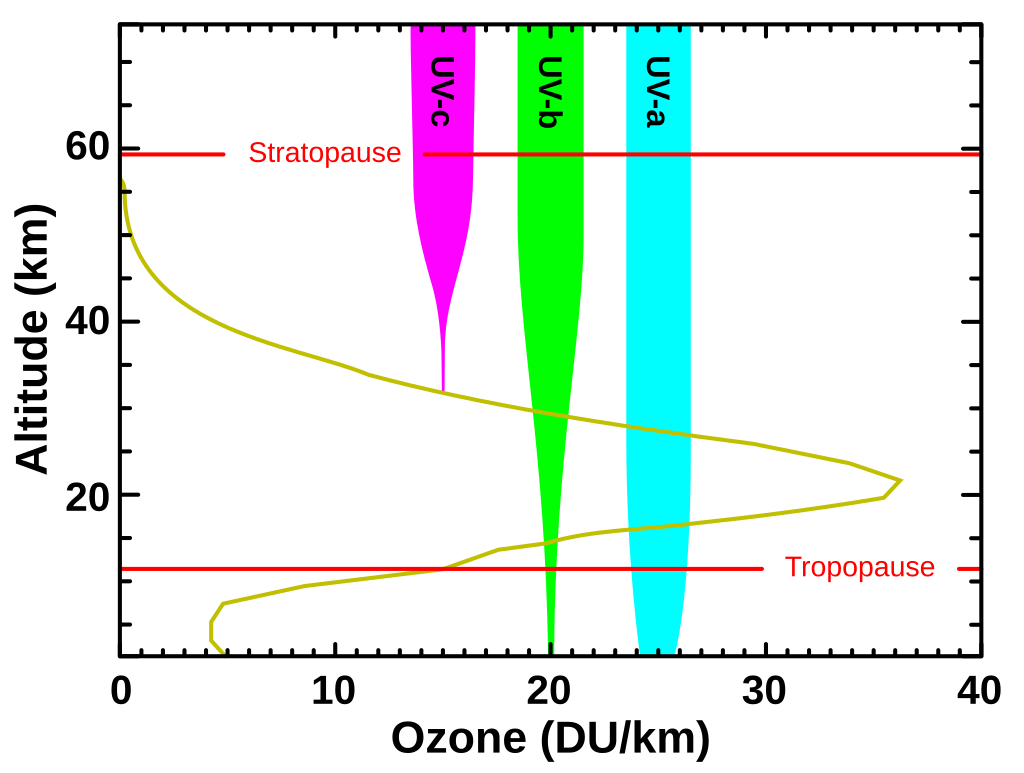
Ninety percent of the Earth’s ozone is found in the lower stratosphere, known as the ozone layer. Despite its importance, the ozone concentration is relatively low—ranging from two to eight parts per million. If all this ozone were compressed to sea level under normal atmospheric pressure, it would form a layer only about 3 mm (0.12in) thick.
That’s still sufficient to protect our planet from UV radiation. The ozone layer absorbs between 97% and 99% of UV rays. Specifically, it filters three ranges of UV radiation: the most harmful, with wavelengths of 100–280 nm; then moderate UV rays, with wavelengths of 280–315 nm, which cause us to tan and increase the risk of skin cancer; and the least harmful, with wavelengths of 315–400 nm, which have minimal impact on our health.
To recap: ozone and oxygen in our atmosphere absorb most of the harmful particles, significantly reduce those rays that affect the skin, and have minimal effect on the 315–400 nm range. So it would be inaccurate to claim that the ozone layer blocks all the ultraviolet radiation.
The ozone hole
We wouldn’t have much to worry about if not for the discovery of an ozone hole in 1985. The phenomenon describes a dramatic decrease in triatomic oxygen molecules in the stratosphere. The depletion was severe enough to be visible over major industrial cities in Europe and North America, raising major concerns about increased cancer risks for the people in these areas.
However, these incidents were short-lived. In contrast, the massive ozone hole over Antarctica turned out to be more persistent. This hole usually forms at the end of the Antarctic winter, around August to September, and can last for several months.
This discovery caused widespread alarm, leading to quick action with the Montreal Protocol, which successfully tackled smaller ozone holes. Despite this, the large hole over Antarctica only shrank a little.
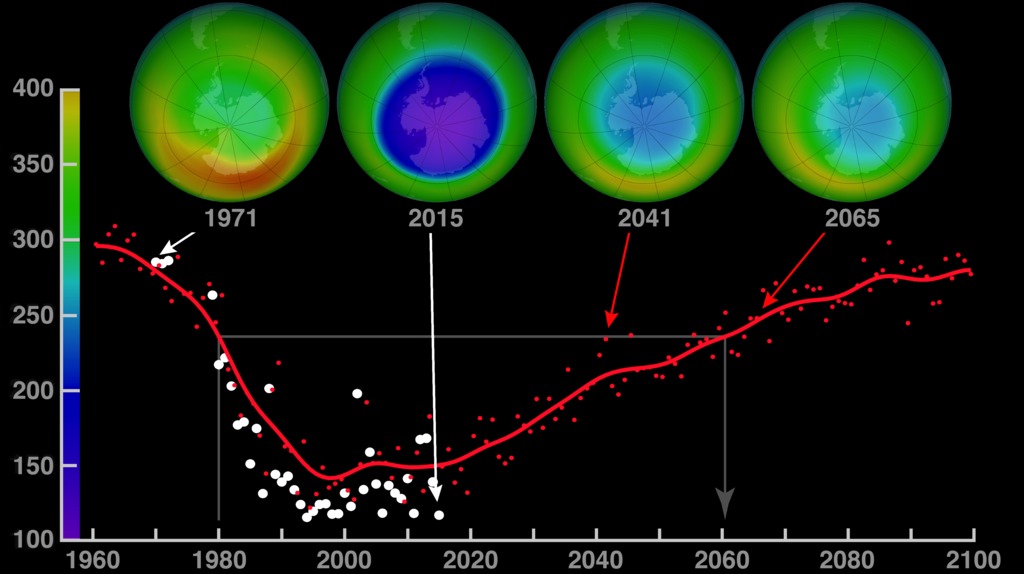
Some argue that the ozone hole might have always been there. But as we’ve been monitoring the atmosphere over Antarctica since the 1920s, we can see from the records that the hole only emerged in the 1970s.
What destroys ozone?
Ozone is naturally unstable and can break down due to many factors. For instance, it can fall apart just by interacting with oxygen. It also gets destroyed when it comes into contact with ozone oxide, chlorine, or bromine.
The latter two elements are particularly linked to human activity, as they are components of chlorofluorocarbons, commonly known as freons—chemicals used in fridges and air conditioners since the mid-20th century.
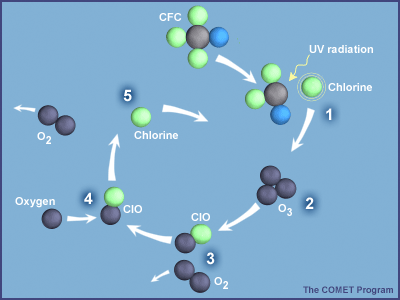
Although it might seem like these pollutants should only affect the areas where they’re emitted, CFCs are actually carried by winds throughout the atmosphere. So, why do they have such a big impact over Antarctica? There are a few reasons for this.
The first and most significant factor is the polar night. For six months of the year, Antarctica remains in total darkness, so ozone gets destroyed by natural processes without getting a chance to be rebuilt.
Another factor is that Antarctica is a landmass surrounded by ocean, unlike the Arctic. This setup creates a huge vortex in winter that keeps outside ozone from reaching the South Pole.
As a result, the ozone layer in these areas becomes especially vulnerable to chlorine and bromine buildup in the atmosphere. These substances destroy ozone molecules, and since there’s no way for new ozone to form quickly, the damage accumulates. By the end of winter, this process hits its peak.
Scientists believe that if we stick to the Montreal Protocol, the ozone hole over Antarctica will eventually close completely. But it’s going to take several decades for that to happen.


